Background:
The AML EBMT Cytogenetic Risk score is a new prognostic model recently published (Canaani et al. Leukemia. 2019 Aug;33(8):1944-1952; Nagler el al. Am J Hematol. 2020 Jun 12) combining cytogenetics and FLT3ITD status for AML patients in complete remission (CR) at transplant time. The AML EBMT Cytogenetic Risk score is prognostic for leukemia-free survival (LFS), overall survival (OS), GVHD-free/relapse-free survival (GRFS) and cumulative incidence of relapse (CIR). In our centre, we frequently offer in-vitro partial T-cell depleted graft (pTDEP) for patient in CR to decrease morbidity and mortality associated with graft-versus-host disease (GvHD). Currently, The AML EBMT Cytogenetic Risk score has not been evaluated in this population.
Aims:
We investigate the impact of the AML EBMT Cytogenetic Risk score for 3 years OS, LFS, GRFS, CIR and NRM in a cohort with patients allografted with pTDEP graft.
Methods:
All consecutive ≥18 years patients who received a first allograft for AML between 2008 and 2018 with data available to determine The AML EBMT Cytogenetic Risk score in CR at transplant time were included. OS and LFS were investigated with the Kaplan-Meier method and we used the cumulative incidence estimator as defined by Fine and Gray to calculate CIR (with NRM as competing event), NRM (with relapse as competing event) and GvHD (with relapse as competing event).
Results:
135 patients were included, median age at transplant time was 56 years (range: 19-74), 44% were female, median Karnofsky index was 90 (80-100). 21% of graft were from HLA identical, 57% from matched unrelated donor, 10% from mismatched unrelated donor and 12% from haploidentical donor. Stem cell source was peripheral blood in 89% and bone marrow in 11%. Partial in-vitro T-cell depletion (pTDEP) was performed in 40% of HSCT. Reduced-intensity (RIC) was performed in 62%. Median follow-up was 3.1 year (range 1.3-10 years) for living patients.
Among the 135 patients, 4 (3%) were assigned in the favourable (Fav), 58 (43%) in the intermediate/FLT3wt (Int/FLT3wt), 36 (27%) in the intermediate/FLT3ITD (Int/FLT3ITD), and 37 (27%) in the adverse (adv) risk group.
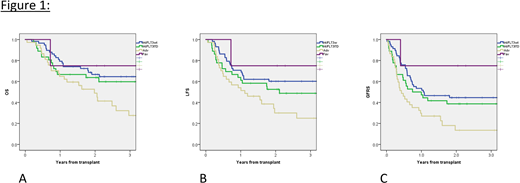
3-years OS for Fav, Int/FLT3wt, Int/FLT3ITD and Adv was 75% (32-100%), 65% (52-77%), 60% (43-77%) and 28% (10-45%), respectively (p=0.033) (Fig 1A). 3-years LFS for Fav, Int/FLT3wt, Int/FLT3ITD and Adv was 75% (95%CI: 32-100%), 60% (47-73%), 49% (32-66%) and 25% (8-42%), respectively (p=0.028) (Fig 1B). 3-years GRFS for Fav, Int/FLT3wt, Int/FLT3ITD and Adv was 75% (32-100%), 45% (31-58%), 39% (22-55%) and 14% (0-27%), respectively (p=0.008) (Fig 1C). 3-years CIR for Fav, Int/FLT3wt, Int/FLT3ITD and Adv was 0%, 22% (11-33%), 31% (15-47%) and 56% (37-75%), respectively (p=0.02). 3-years NRM for Fav, Int/FLT3wt, Int/FLT3ITD and Adv was 25% (95%CI: 0-75%), 17% (7-28%), 21% (6-35%) and 17% (2-32%), respectively (p=0.92). 3-years grade 2-4 aGVHD for Fav, Int/FLT3wt, Int/FLT3ITD and Adv was 0%, 35% (22-47%), 19% (2-36%) and 42% (25-58%), respectively (p=0.46). 3-years cGVHD for Fav, Int/FLT3wt, Int/FLT3ITD and Adv was 25% (95%CI: 0-75%), 17% (6-27%), 22% (7-38%) and 21% (6-35%), respectively (p=0.9). In addition to The AML EBMT Cytogenetic Risk score, variables with a significance in univariate for OS with a p-value ≤0.1 (Karnofsky index [<90 vs. ≥90]; stem cell source [PBSC vs. BM] and donor type [matched vs mismatched donor]) and pTDEP were included in the multivariable model. In multivariable analysis, only the Cytogenetic Risk Score (Fav + Int/FLT3wt: ref; Int/FLT3ITD + Adv: HR: 1.8 [95%CI: 1.1-3.1], p-value 0.03) and Karnofsky index (<90: ref; ≥90: HR 1.8 [95%CI: 1.0-3.2], p-value 0.049] remain significant. Because of the low number of patients in pTDEP (53) and non-pTDEP (82), statistical analysis couldn't be performed specifically in these subgroups but pTDEP had no impact in multivariable analysis for OS.
Conclusion:
In the analysis of our retrospective cohort including 40% of pTDEP patients, we confirm that the AML EBMT cytogenetic risk is prognostic for relevant outcomes (OS, LFS, GRFS, CIR) of HSCT. Similarly with recently published data, we confirm this prognostic model can help physicians and patients in transplant choice and remains valid for patients undergoing HSCT with in-vitro graft manipulation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal